Lithium iron phosphate batteries are part of a group of batteries called lithium-ion batteries. Specifying that a battery is a lithium iron, or lithium iron phosphate, battery implies a host of characteristics which are important when choosing a battery type and provider, says REVOV co-founder and engineering director, Felix von Bormann.
Revov, which is the leader in 2nd LiFe Lithium Iron storage batteries for UPS systems and renewable energy sources, uses Lithium Iron Phosphate batteries, which are a specific subset of a larger group of batteries called lithium-ion.
Lithium iron phosphate batteries (LiFePO4, or as some refer to it, LFP) have many advantages, says Von Bormann. “Revov uses Lithium Iron Phosphate batteries because they have a great energy density and lifecycle, a stable chemistry all while maintaining high thermal runaway and resulting in a long life at low costs,” explains von Bormann.
How it works
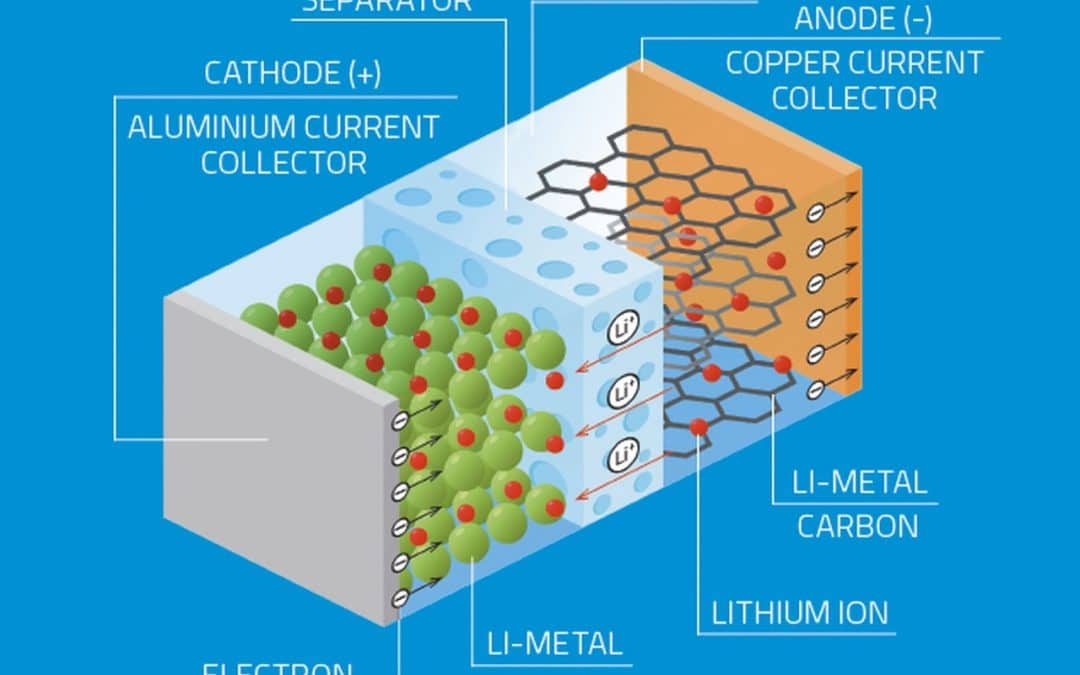
Lithium-ion batteries are secondary cells created from layers of lithium packed with an electrolyte. A lithium battery is formed by four important components namely the cathode, anode, electrolyte, and separator.
The cathode, a positive electrode, determines the capacity and voltage of the battery and is the source of lithium ions. The anode, a negative electrode, stores the lithium ions when the battery is charged and enables the electric current to flow through an external circuit. The electrolyte is formed of salts, solvents, and additives and serves as the channel of lithium ions between the cathode and anode while the separator is the physical barrier that keeps the cathode and anode apart.
The cathode is a metal oxide, and the anode consists of porous carbon thus during discharge the ions flow from the anode to the cathode through the electrolyte and the separator, when charging the battery, the reverse happens the ions flow from the cathode to the anode.
Lithium-ion batteries come in many varieties, although remarkably similar on the surface these batteries vary in performance and the selection of active materials gives them distinctive qualities.
There are six main versions of Lithium-ion batteries namely Lithium Cobalt Oxide, Lithium Manganese Oxide, Lithium Nickel Manganese Cobalt Oxide, Lithium Iron Phosphate, Lithium Nickel Cobalt Aluminium Oxide and Lithium Titanate.
The cathode is what differentiates each battery. For example, the Lithium Cobalt Dioxide is made with a LiCoO2 cathode while the Lithium Iron Phosphate battery is made with LiFePO4 as the cathode. This means that there is a technical difference between Lithium-ion and speaking about Lithium Iron. Lithium-ion references the mode of electrical transfer inside the battery, where ions travelling in the electrolyte are lithium. Lithium Iron is a subset of the family of Lithium-ion batteries.
Despite the characteristics they have in common the different Lithium-ion systems and Lithium Iron batteries are different in terms of their stability, life span and application. Lithium Iron Phosphate has a high current rating and long cycle life, it is more tolerant to full charge conditions and is less stressed than other lithium-ion systems. It has a specific energy of 90/120 watt-hours per kilogram, a nominal voltage of 3.20V/3.30Va, the charge rate of 1C, and a discharge rate of 1-2,5C.
Von Bormann says Lithium Iron Phosphate loses some distance in terms of specific energy per kilogramme, “However, this is not important in a stationary application where weight is less important. The Lithium Iron Phosphate cell is superior in terms of safety and price, over other Lithium-ion battery chemistry, and typically has a higher Life Expectancy and a Higher Specific Power,” he says.